BY LETTER
Chlorine Worlds
aka Chlorogaian Worlds | |
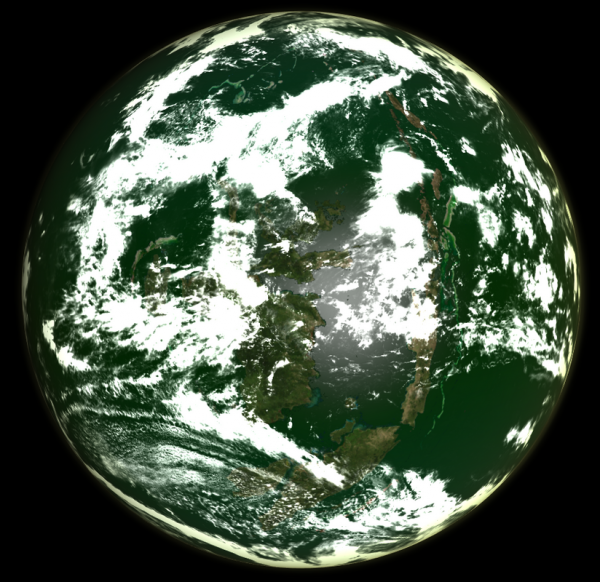 Image from LordOther | |
| Doreen, A chlorogaian world, with a biosphere that both produces excess chlorine and utilises it as a life-supporting element | |
Chlorine worlds are like typical Eugaian worlds, with one important difference: the process of photosynthesis releases free chlorine. Such biospheres can only exist on those rare terrestrial worlds that bear a high percentage of hydrogen chloride in addition to the more usual water. Some believe that the coincidence of a gardenworld with a sufficient supply of hydrogen chloride in that world's hydrosphere is so unlikely that there are no natural chlorine worlds in the entire galaxy. Despite this there are 356 known chlorine worlds and former chlorine worlds in the Terragen sphere of exploration alone, and it is believed that many more remain undiscovered along the periphery. Though they are vastly outnumbered by more conventional life bearing worlds, this number is orders of magnitude too high for them to be a natural phenomenon. It appears that every one of these biospheres, living or fossil, is due to the agency of a mysterious clade or group of clades known popularly as the Halogenics. The Halogenics left few traces other than the chlorine worlds themselves, all of which were created over a relatively short period of time 780 million years ago. Though little is known of their nature and nothing at all of their motives their legacy is extensive.
Planetary Formation
The typical processes of nucleosynthesis in stars create more even numbered elements than odd, and more light elements than heavy ones. Chlorine is a relatively heavy element, and odd numbered. On the average it is over 20,000 times less common than oxygen, the element that is normally released by photosynthesis on inner system worlds that bear life. Nevertheless, there are some small regions of the galaxy in which the interstellar medium is enriched in chlorine. This is particularly true where there are second and third generation stars, which have a higher "metallicity" (percentage of elements heavier than hydrogen). In the case of the chlorine worlds, the interstellar medium from which a star and its attendant planets have formed must also be especially enriched in what are known as alpha-process elements (sulphur, chlorine, argon, calcium, titanium, and chromium). If that relatively rare nucleosynthetic process was common enough in the stars that formed the parent material, then a chlorine world may be possible.Even if the original materials are chlorine-enriched, the process of planetary formation must follow a fairly uncommon series of events to create a chlorine world. Hydrogen chloride is a volatile substance compared to water, and unlike water is not often combined wholesale with silicates (hydrated "wet" silicates are a major source of water in the formation of terrestrial planets). The band of material cool enough to retain sources of hydrogen chloride and yet warm enough to allow the formation of a rocky planet is very narrow. Inside that narrow band a planet retains only water in its hydrosphere (though there may be unusual concentrations of more refractory chloride minerals); outside that band gas giants or iceball worlds are the rule. Most often, a chlorine world is formed at the outer edges of a system's life zone, or even outside it. As the star ages and warms, or as the dynamic between the interplanetary material and the planets brings orbits closer to the primary, a chlorine world becomes possible.
Planetology
The unusually high metallicity of the original material means that there are more heavy elements with higher melting points in the inner regions of a system with chlorine worlds. With more material in a solid state, this means that more is available for the formation of planets and moons. Chlorine worlds therefore tend to be much more massive than typical inner-system planets, and so do their moons and any neighbouring rocky worlds. The smaller chlorine worlds tend to mass as much as the earth, and the most massive known have ten times earth's mass. The exceptions are usually moons of inner system gas giants, or even large moons of other chlorine worlds; these may be relative lightweights.The crust of a chlorine world is relatively rich in metals of all kinds, and (because they are "sister" elements of the abundant chlorine), has unusually high proportions of calcium, titanium, and chromium. The other two major alpha process elements are sulphur and argon, but the former has a strong affinity for the planet's nickel-iron core and the latter remains a gas and is not incorporated into the planet at all. Chlorides are among those elements concentrated in the crust and hydrosphere, further increasing their relative abundance. The planet's chlorides are most often sylvite, halite, simple salts of copper or iron, or more complicated chlorides. However, if the interplanetary medium was not too hot during its formation there is also a large quantity of hydrogen chloride. This is emitted from volcanoes in the form of hydrochloric acid, which helps to form a significant fraction of the planet's hydrosphere and atmosphere. The water and hydrochloric acid together are strong weathering agents: far more so than on a typical water-world. They react with most silicate minerals, excepting a few resistant forms such as quartz and muscovite, to form clays, halide salts, aluminum oxides and hydroxides, and silica. Exposed rock weathers away quickly, especially if it is a low-silica rock such as basalt. Granites last longer before they crumble away. Most of the planet's surface is coated in sand or mud, and there are extensive deposits of acid-resistant minerals such as rutile, gold, corundum, cassiterite, and diamonds. Though water and hydrochloric acid are consumed in these reactions, they are re-constituted when sedimentary rocks are buried and heated in the geologic cycle, and are re-emitted by volcanoes and hot springs.
Typical chlorine worlds have numerous mountains and volcanoes, and many relatively fast-moving tectonic plates. The mountains themselves are usually low: a hot crust and mantle are relatively plastic, and this together with the typical strong gravity of these massive worlds does not permit great initial heights. Also the heavy gravity and acidic rains speed erosive forces. Often there is relatively little land, because the hydrosphere is usually quite extensive. Shallow epicontinental seas are the general rule, and deep ocean basins are rare (again because of the weak crust and heavy gravity). Hot springs are ubiquitous on all but the oldest chlorine worlds. There are many exceptions to these general rules, of course, particularly on the smaller more marginal chlorine worlds.
 Image from Chris Shaeffer | |
| The purple xenoflora and green skies of Chorus | |
The atmosphere of a mature chlorine world consists primarily of oxygen and nitrogen, with a significant fraction of chlorine (about 5%). The chlorine is found mostly in the lower atmosphere, because it is heavier than the other primary gases, and it has a higher relative abundance over dry areas because it eventually reacts with water to produce hydrochloric acid again. Only the highly efficient and energetic photosynthetic activity of the plants and bacteria maintains the supply of free chlorine. The atmosphere is not clear but has a yellow-green tinge from chlorine and from various forms of chlorocarbons; on some chlorine worlds a thick atmosphere encourages the growth of airborne life forms as well, which may give the lower atmosphere a murky, smoky look. Clouds consist in varying proportions of water or hydrogen chloride droplets. The weather is complicated by the fact that hydrochloric acid evaporates more easily than water, and freezes much less easily. Rain is always acidic, but the concentration varies considerably. Ice and snow are rare, and even extremely cold bodies of water rarely freeze. When they do, the acidity rises sharply beneath the water ice due to the removal of water relative to hydrochloric acid.
 Image from Steve Bowers and John M Dollan |
Though they represent a relatively small percentage of the atmosphere, the lighter chlorocarbons (such as carbon tetrachloride, chloroform, and methyl chloride) have a significant effect. Some are strong greenhouse gases; this is one of the things that allows chlorine worlds to exist further from their primary than more typical garden worlds. The other effect is less favourable: they react with ozone, and prevent the formation of an ozone layer. Therefore ultraviolet light from the primary reaches the surface, rendering life on land or near the ocean's surface hazardous. Most chlorine worlds are found around K or even M type stars, but the few around G type stars may have rather restricted ecosystems.
Biochemistry
Life forms on all known chlorine worlds exhibit the same fundamental biochemistry. This, together with some consistent aspects of cell morphology, is considered to be a strong sign of common descent. As on more typical worlds, life on chlorine worlds produces chemical energy from sunlight by using it to reduce available hydrogen-bearing compounds. Just as on terragen-style worlds, the most common hydrogen donor is water, simply because it is so abundant. This form of photosynthesis releases oxygen. However, chlorine worlds also have a large stock of hydrochloric acid, and photosynthetic organisms also make use of that resource, and release chlorine. The splitting of water and the splitting of hydrochloric acid both release hydrogen ions and high-energy electrons, which are then used to produce carbohydrates and other organic compounds. The usual carbon source is carbon dioxide. There are therefore two dominant kinds of photosynthesis on chlorine worlds:2HCl + CO2 ---> CH2O +Cl2
in which hydrochloric acid and carbon dioxide are consumed and organic compounds and chlorine produced, and
H2O +CO2 ---> CH2O + O2 the far more common process familiar from Terragen and similar biochemistries.
Most photosynthetic organisms actually prefer to use hydrochloric acid if it is available, but the availability of water makes it the more common donor. Release of chlorine ultimately often leads to oxygen in the atmosphere in any case, since the chlorine reacts with water to release oxygen and produce hydrogen chloride again. The combination of the various oxygen and chlorine releasing photosynthetic pigments is typically purplish-black to human eyes. In the light of a typical K type star, that colour is quite nearly black. Respiration on chlorine worlds is the reverse of photosynthesis, and most organisms are capable of using either chlorine or oxygen as fuel. So, an animal on a chlorine world breathes out not only carbon dioxide and water but also hydrochloric acid. Chlorocarbons are abundant in the biosphere, and participate in many biological pathways (unlike the case on more typical garden worlds, such as Earth, in which natural chlorocarbons are present but relatively rarely produced by biological activity). Some particularly resistant chloride polymers are used by land-dwelling life forms to protect themselves from excessive concentrations of hydrogen chloride or from pure water, either of which is harmful to their tissues.
The abundance of heavier metals means that not only is life on chlorine worlds immune to poisoning from these but it has also become dependent on them. The photosynthetic and respiratory pigments, and many enzymes, are dependent on a variety of metallic ions that are rather rare on more conventional garden worlds. Often the shells and casings of chlorine life are made of unusual acid-resistant elements and compounds (gold is a common component).
All life on the known chlorine worlds shares the same genetic code. The detailed chemistry differs, but the overall pattern is rather startlingly similar to that of Terragen life: a double-stranded polymer remarkably similar in structure and function to DNA.
Life on Chlorine Worlds
On most chlorine worlds, swamps and shallow seas are the dominant biomes, due to underlying geology. Life on the land is exposed to some significant hazards and difficulties that are unique to chlorine world environment. These include heavy gravity, exposure to whatever ultraviolet light the local star produces, and a very wide range in local acidity. The last factor is perhaps the most difficult for life. Plants usually have waxy leaves and bark composed of resistant organochloride polymers. Animals have similar resistant coatings, and may become temporarily dormant if local conditions are particularly poor. Tree-like life does not reach impressive heights, except for those organisms that buoy themselves up with bladders of light gases. This allows them to survive without massive structural supports. The habit of developing balloon-like supports has lead to the extensive development of airborne life, from tiny spores to gigantic mats of floating vegetation. While large flying forms are often quite rare due to the heavy gravity, the dense air of a typical chlorine world encourages numerous insect-like forms. The atmosphere of some chlorine worlds is smoky with analogs of animal, plant, and fungoid life.Though all chlorine worlds share a common set of biochemical pathways, the multicellular forms vary widely from one world to the next. It appears that the Halogenics either designed separate multicellular forms for each world, or that multicellular life evolved separately in each instance. Fossil evidence suggests the former of these. The reasons for such a strategy are, of course, completely obscure.
Terragen Impressions
To Terragens accustomed to earth-like worlds, a typical chlorine world is bizarre and extremely uncomfortable. Most are extremely hot due to greenhouse effects (daytime temperatures of 30° to 50° Celsius are typical), and earthquakes are frequent. The gravity is oppressive, and light at the surface is dim and greenish. Not only is the chlorine content of the atmosphere toxic to most bionts, but the numerous chlorocarbons (which include small quantities of chemicals such as chloroform and mustard gas as well as a number of more subtle toxins such as PCBs) are cumulatively deadly. Even small quantities of chlorine world atmosphere leaking through airlocks are unpleasant. The rains of strong hydrochloric acid and the weaker but still deadly acidic seas are also harmful not only to bionts but to vecs, as well as to many aspects of standard terragen technology. Standard nano may fail to function entirely, though specialized versions operate perfectly well, and diamondoid eventually erodes. In addition, terragen life and technology must be protected from high concentrations of unusual metals and semimetals such as selenium, arsenic, and various rare earths, most of which are at levels that would be toxic to terragen bionts. The life forms are physically strong, and their ordinary bodily fluids are strongly acidic. The vegetation is low lying, and to typical terragen eyes is dark or even black. The air and water are thick with life, sometimes unpleasantly so. Chlorine worlds are not popular tourist destinations. Image from LordOther | |
| Tytalus, a Chlorogaian near Sol | |
Chlorine Worlds in the Terragen Sphere
Chlorine worlds are found scattered throughout the entire volume of terragen space. In the time since their discovery nearly all of the living chlorine worlds have been placed under protection by one or another of the Caretaker Gods or by transapients with similar mind-sets that have been so assigned by the nearest meta-empire. In the Sephirotic meta-empires this is a fairly civilized process, and involves invitations or appointments of such guardians. Elsewhere the Caretakers have sometimes used force to displace would-be colonists in the system. Colonists on the Periphery avoid moving into a system that contains a chlorine world, since in some cases the Caretaker will place not only the chlorine world itself but the entire stellar system under the Compact of Eden regulations.Several Chlorine Worlds have been detected beyond the Terragen sphere of expansion by the Argus Array, and the ultimate extent of these worlds remains unknown. Notable chlorine worlds include Doreen (the site of a chlorine-breather civilization, now extinct), Tytalus and Outland (the first two chlorine worlds discovered by Terragens), and Chorus, where the only extant sophont chlorine breathing species was provolved) by Terragens from a presapient species.
Related Articles
- Chorus
- Chorus - Xenobiology
- Doreen/Doreens
- Halogenics
- Jade Chime Singers
- Non-Luminary World Classification Scheme
- Outland
- Tytalus
- Xenobiochemistry
- Xenobiologist
- Xenobiont
- Xenobiota - Text by M. Alan Kazlev
The totality of non-terragen life on a specific planet or non-terragen ecosystem. - Xenologist
- Xenophotosynthesis
Appears in Topics
Development Notes
Text by Stephen Inniss
Original version by Anders Sandberg
Initially published on 30 July 2000.
Original version by Anders Sandberg
Initially published on 30 July 2000.