BY LETTER
Vitriolic Type Worlds
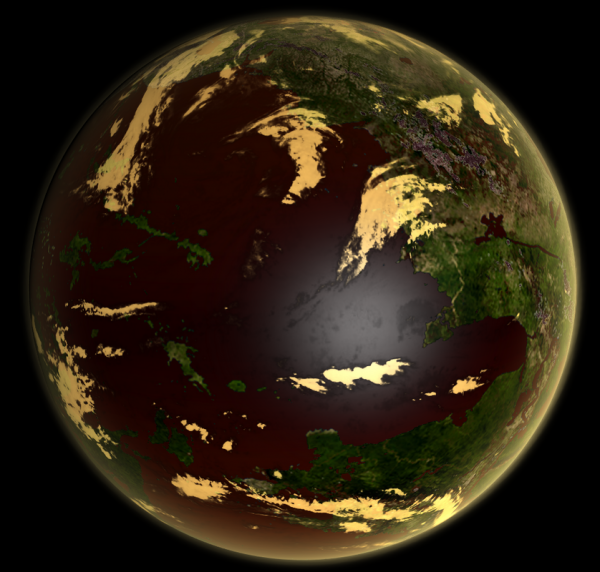 Image from LordOther | |
| A Vitriolic type world, with a biosphere that both produces and utilises atmospheric sulphur compounds | |
Planetary Formation
As sulfuric acid is not a common chemical, a terrestrial world will not simply form with oceans of anhydrous sulfuric acid, they must be formed after planetary formation by an unusual set of conditions. First, a world must possess a great deal of sulfur and the sulfur must be concentrated at the surface. This is the only relatively simple condition. Old Earth for example is approximately 13% sulfur, compared to ~30% oxygen and worlds with greater sulfur concentrations are not uncommon. As to the concentration of sulfur at the surface, this is easily accomplished via the volcanic activity of worlds which are young, subject to strong tides or possess large amounts of radioactives in their cores. It is important that such activity not be so powerful as to leave the planet wholly molten for too long, such a state is conducive to allowing sulfur to bond with chalcophilic elements such as iron, leading to much of the sulfur being bound in the planetary core.Second, a world must possess water, ideally, enough to form oceans. Third, the world in question must be subject to intense photodisassociation of its water supply. The splitting of water molecules will yield a primal CO2 atmosphere rich in oxygen and, for a limited time, hydrogen. The sulfur and oxygen will combine to form sulfur dioxide and then sulfur trioxide, the latter will then combine with this/any remaining water to form sulfuric acid. The free hydrogen will eventually escape, but some will be preserved as part of the sulfuric acid and what little water that remains. Over the rest of the planet's lifetime, the amount of sulfuric acid tends to be remain balanced; subduction brings some sulfuric acid to the mantle where it is broken down, volcanoes release hydrogen sulfide, sulfur dioxide and water vapor which will react with oxygen and one another to recreate the sulfuric acid lost in the mantle.
The latter condition is what really makes these worlds so rare, there are multiple fine balances that must be maintained. If the photodisassociation proceeds too quickly, the entire world's water supply will be destroyed before the sulfuric acid oceans form and lead the world to a runaway greenhouse effect, likewise if the world possesses too little water. If a world possesses too much water, the sulfuric acid will never overcome it and will remain, if anything, a minor constituent of the oceans, continually renewed by volcanic outgassing of sulfur dioxide as it is destroyed by reactions with water. Also, a world in such a warm orbit with too much water runs a high risk of a runaway greenhouse effect, the same applies if its primal CO2 atmosphere is too thick. For these reasons, these worlds tend to be naturally found around G and F-type Population I stars.
 Image from Steve Bowers | |
| LiuShan, a vitriolic type world with an average temperature of 300°C, is much hotter than an Earth-like planet, yet it holds a complex biosphere | |
Planetology
Chemically, Vitriolic worlds somewhat resemble hot springs occasionally found on Gaian worlds, where sulfuric acid sometimes can be found. In both cases, the sulfuric acid creates intense chemical weathering, as it dissolves and reacts with most common minerals and metals. The acidity of the oceans, seas, rivers and lakes also prevents carbonates from being stable, mandating that CO2 be bound up by other minerals or compounds to avoid a runaway greenhouse effect. On most worlds, this is accomplished by organically-created minerals. It is also rare for these worlds to possess dense atmospheres, helping prevent excessive CO2 buildup.Quartz, often the most common mineral in the continental crust of Gaian worlds, is among the groups of minerals that are stable in the presence of sulfuric acid, as are many of the clay-like minerals left over from sulfuric acid's destruction of feldspars. Corundum, known for its gem forms such as rubies, is also stable and may be found. Metal sulfides and many normally rare sulfur minerals are very common. Arid regions will often possess deposits of Anhydrite, anhydrous calcium sulfate, in rarer cases, the hydrated variant known as Gypsum and other hydrated minerals may also be present. A few other salts are also stable, but sodium chloride is not among them, sulfuric acid reacts with it to form sodium bisulfate, which will be dissolved, and hydrogen chloride gas, some of which will remain as a gas while some will be dissolved as well.
The sulfuric acid seas and oceans of these worlds are extremely salty due to the acid's tendency to dissolve most minerals and ionize most metals. It is not uncommon for sulfates to precipitate in cooler temperatures because of this. It is important to note that despite sulfuric acid's great dissolving power, non-saline lakes and rivers may exist as eons of erosion have scoured the inlands of most everything the acid can dissolve, what little is left is often secured by local plantlife. The seas, lakes and rivers also have a rather odd appearance because of sulfuric acid's viscosity, which is 27 times greater than water and somewhat oily/syrupy in appearance, a trait which gave the acid its ancient name, Oil of Vitriol. This viscosity tends to mute waves and this is exacerbated by the acid's density, which is 1.84 times that of water, thus demanding more energy to move a given volume. This high density combined with acidity tends to mute elevations on these worlds by intense chemical and mechanical weathering. Also, while pure sulfuric acid is colorless, the great amount of dissolved salts and minerals in saline oceans and seas often make them brown if not stained by local lifeforms.
The atmospheres of these worlds are actually not particularly unusual to Terragens except for their small volume of sulfuric acid vapor and extreme temperatures. Primal atmospheres will be largely CO2 and nitrogen, while mature worlds with flourishing biospheres will be composed largely of nitrogen and oxygen with trace amounts of CO2, sulfuric acid and water vapor. However, non-Thiogen examples of mature Vitriolic worlds with higher, even dominant, partial pressures of CO2 are not relatively uncommon. Average planetary temperatures usually fall between 250 and 330°C, a range including all Thiogen worlds, lower ranges are known on a minority of natural examples. Due to sulfuric acid's very wide liquid range, approximately 10 to 338°C for pure solution and even greater in practice, a Vitriolic world possessing even sparse high altitude or polar frozen sulfuric acid is extremely rare. This has the effect of entirely preventing ice ages (though not necessarily shifting climates) and avoiding the complications involved with an ice which sinks in its liquid form.
As the sulfuric acid vapor is largely colorless and these worlds tend to orbit G and F-type stars, their skies are often a pale shade of blue or green, some may even appear white. Many worlds will tend toward the green due to the presence of sulfate aerosols. The sulfates not only scatter light more strongly than the nitrogen, oxygen and sulfuric acid vapor, but they are also effective condensation nuclei, encouraging the production of many small sulfuric acid droplets in the air, which scatter light more effectively than a smaller number of larger droplets. This also leads to greater production of clouds and increases the duration of the clouds. Both the sulfates and sulfuric acid vapor of the clouds have a very high albedo, making these worlds bright and brownish from orbit. This high albedo combined with the necessity of a very hot climate mandates that these worlds occupy very warm orbits, those averaging solar fluxes of approximately 1,700 to 3,800 watts/meter2, roughly equivalent to that of Venus in Solsys.
Biochemistry
The biochemistry of these worlds is very different than that of Terragen life, it is based on silicones, alternating chains of silicon and oxygen. In Terragen conditions, silicones do not form naturally, silicates are too stable. On Vitriolic worlds, however, the extreme heat and acidity are capable of breaking some silicates into basic units of silicon and oxygen, which are hypothesized to then be able to combine into silicones. It has never been directly observed, and there are many xenobiologists who believe it all but impossible and therefore that even the 28 known "natural" Vitriolic worlds must be the product of sophont intervention. There is no hard evidence in support of this hypothesis however.What gives these silicones the complexity necessary to support organic life are the organic side groups attached to the silicon atoms. These side groups are based primarily on the usual elements found in organic life; carbon, hydrogen, oxygen, nitrogen, phosphorous and sulfur, but other elements are also sometimes employed. In relatively simple molecules, the chains may be short and the side groups constant, but there is an almost infinite variety of molecules possible if the chains are made different lengths and their side groups varied. In addition to the silicones, alkanes, the simplest hydrocarbons, are also often employed as they are stable in sulfuric acid. A number of other traditional CHON organics are ionized by protonation (addition of hydrogen nucleus) but remain intact and are therefore frequently used in roles where ions are useful or critical, such as nervous systems and minor biochemical pathways. The trace levels of water on these worlds are sometimes used for various purposes in microorganisms.
Many vitriolic worlds have photosynthesizing lifeforms. The exact biochemical pathways can vary, but the basic process is similar on most of these worlds and somewhat familiar. Sunlight drives cellular processes which combine CO2, sulfuric acid and basic silicones into energy-rich silicone polymer "sugars" and release free oxygen. Cellular respiration is, of course, the opposite; silicone "sugars" are "burned" with oxygen to produce CO2, sulfuric acid and waste silicones. The silicone substrates are commonly solid, but sometimes liquid, and generally are not produced in sufficient quantities to raise removal difficulties even for complex multicellular lifeforms.
Sulfur compounds are nearly always of great abundance and importance in Vitriolic biochemistry, owing to their chemical usefulness and great abundance in the environment. Metals are also much more frequently utilized than in Terragen biochemistry owing to the great affinity of sulfuric acid for dissolving them. It should also be noted that the extreme heat of Vitriolic worlds is not an obstacle for local life, it is nearly always a requirement. Many reactions are dependent on the high energy such heat brings, even with the assistance of enzyme-equivalents. As such, temperatures much below 100°C begin to slow down most reactions a great deal.
Life on Vitriolic Worlds
On the 28 known naturally-occurring vitriolic worlds, the biospheres are usually somewhat primitive. Twelve possess oxygen-producing bacteria-equivalents, 14 remain CO2-shrouded worlds with chemosynthetic life and the remaining two have advanced as far as very primitive terrestrial eukaryotic and multicellular organisms. The vast majority of known Vitriolic worlds, however, are the Thiogen ones, ecoformed 560 million years ago.Nearly all of the latter possess advanced eukaryotic multicellular biospheres and while evidence shows all were originally given largely identical stocks, over half a billion years of evolution has created so much incredible variety it takes detailed analysis of genetics and embryology to be sure they indeed share a common descent. Only a handful of constant obvious points remain among Thiogen worlds; plant-life is usually dark red and vertebrate-like animals (where present) possess a single spinal column, laterally-opposing hinged jaws, four eyes and six limbs rather than the Terragen norm of four.
Terragen Impressions
To most Terragens, Vitriolic worlds are an odd combination of the hellish and the friendly and familiar. They are hotter even than To'ul'h worlds and their oceans are strongly acidic, yet their skies are blue or green and life-thrives in a very Gaian way with surface or liquid-bound plant analogues turning the light of suns into chemical energy and free oxygen that drives often-complex ecosystems. None but an extensively modified and/or protected Terragen could stand on such a world and live longer than a few moments, but these worlds hold the natural beauty of any Garden World, a beauty given greater depth for the unusual chemistry driving it all and the long-extinct sophonts who ecoformed the majority of them.Vitriolic Worlds in The Terragen Sphere
Vitriolic worlds are found scattered throughout the Terragen Sphere, all of the Thiogen ones are at least 2,000 light years coreward of Solsys. Due to their highly unusual and interesting nature, most of them, including even some of the primitive natural ones, have been under the protection of Caretaker Gods or similar transapients for thousands of years. The Argus Array has detected thousands more Vitriolic worlds beyond the Terragen Sphere, most of which are undoubtedly possess Thiogen-normal biospheres, based on the detection of the signature red pigment.Related Articles
Appears in Topics
Development Notes
Text by Matthew C. Johnson
Initially published on 27 January 2009.
Initially published on 27 January 2009.